
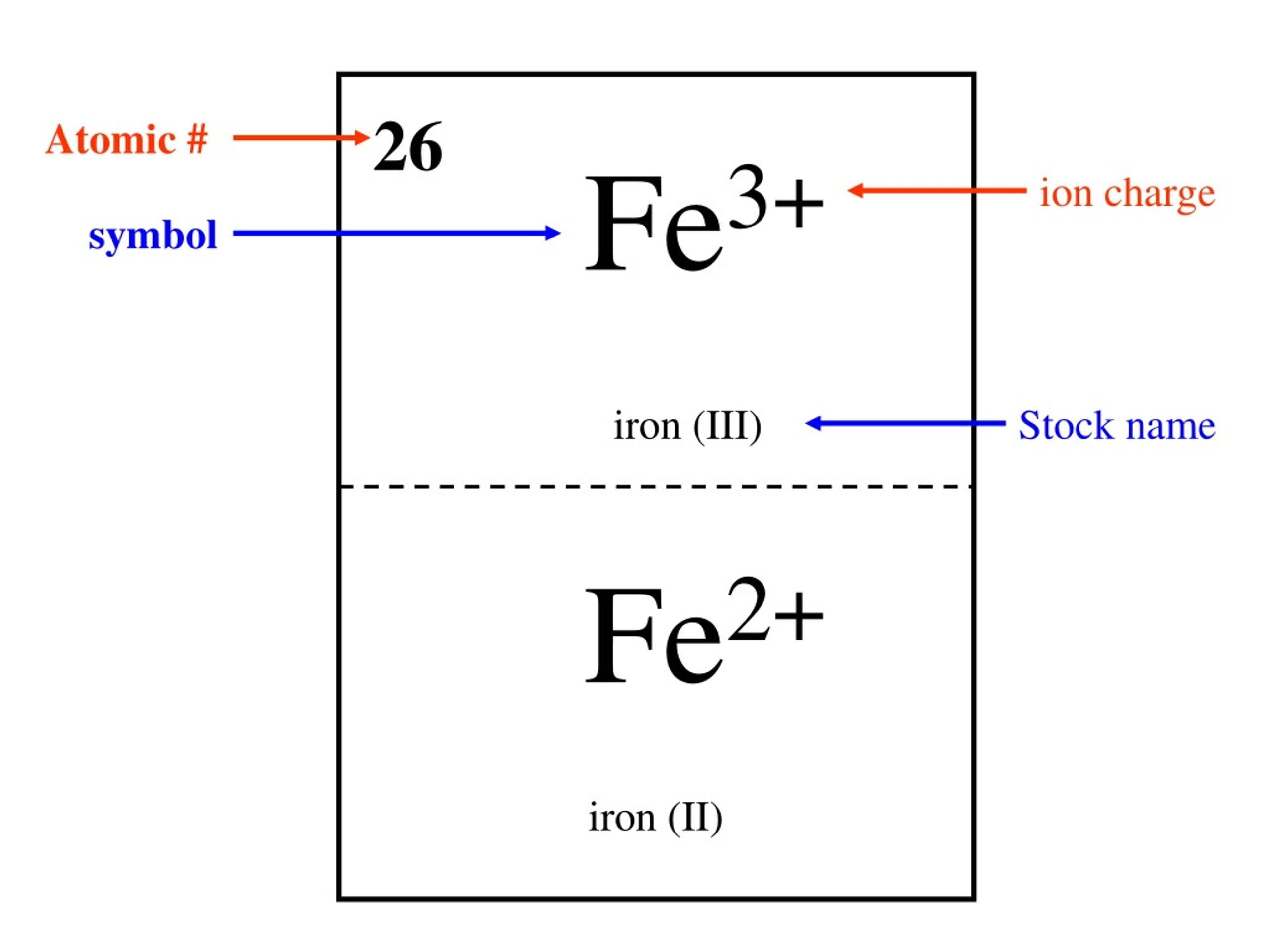
Siegfried Pohl, Ulrich Bierbach, Wolfgang Saak "FeI3SC(NMe2)2, a Neutral Thiourea Complex of Iron(III) Iodide", Angewandte Chemie International Edition in English (1989) 28 (6), 776-777. (III) bromide 4 FeI2 Fe+2 I-1 Iron (II) iodide 3 FeI3 Fe+3 I-1 Iron (III). If the iron(III) iodide were to be formed in a process that avoids the use or generation of the separate ions, it would more likely be sustained.Ĭompare these results with cerium(IV) chloride. Chapter 3 Ionic Bonds Science 9 WORKSHEET Multivalent Metal Ions KEY Name. Diiodotetracarbonyl(II) iron was photochemically reacted with iodine in n-hexane medium to yield the product: It was the year 1989 that the synthesis was somehow achieved. Simple Structure Advanced History Comment on this record 3D Iron (III) ion Molecular Formula Fe Average mass 55.843 Da Monoisotopic mass 55. There are three main steps for writing the net ionic equation for Fe(NO3)3 + KI FeI3 + KNO3 (Iron (III) nitrate + Potassium iodide). Its thermodynamic limitation was known in aqueous medium, so its synthesis was thought to be carried in non-aqueous medium. There was a possibility of its existence indicated by the fact that hydrated ferric oxide dissolves in hydriodic acid, yielding a brown solution. Iron(3+) triiodide FeI7 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. As it is pointed out in other answers and in literature, it is indeed thermodynamically unstable and its reaction synthesis has unfavorable pathways. Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF 3 (H 2 O) x where x 0 or 3.


 0 kommentar(er)
0 kommentar(er)
